Chaperones Conducting the Orchestra in Cellular Assemblies
Researchers at Ashoka University have observed that molecular chaperones can modulate the mechanical stability of a focal-adhesion protein (Talin) and subsequently affect cellular behaviour.
Ceaseless interactions with the basement membrane (a type of specialized extracellular matrix – ECM) allow cells in our body to migrate effectively across tissues. The migration of cells is tightly mediated via a force-driven brake mechanism displayed by large cellular assemblies called focal adhesions (FA). These multi-protein complexes comprise of more than 100 proteins, and mechanical linkages within these complexes enable proper transmission and transduction of mechanical force through the cells. Any distress within this nexus of linkages can oftentimes contribute to a multitude of diseases.
As a result, it is important to investigate various factors that could modulate the force-response of these large assemblies of proteins. Furthermore, given the relative contribution of each protein (that forms focal adhesions) towards cellular migration, evaluating the force-response of each of these proteins also proves to be an important step towards better comprehending the larger orchestra at play. The study, published in Nature Communications Biology, led by Prof Shubhasis Haldar at Ashoka University have pioneered in providing insights into a plausible single-molecule framework to determine the force sensitivity of FA proteins.
Force sensitivity, in simple terms, is defined as the capability of the protein to withstand a certain amount of force while performing its biological functions. Since proteins predominantly exist in two states – folded and unfolded – a folded protein molecule can unfold depending on the magnitude of force that it experiences. Intrinsically, protein molecules tend to remain in their folded states, but a higher mechanical load can lead to the unfolding of these proteins. This mechanical response of proteins establishes a mechanical switch that mediates its interactions with other proteins, and thereby influences cellular behaviour.
Given their involvement in almost every cellular process, the functional and structural maintenance of proteins is of utmost priority to the cell. Quite like its literary counterpart, molecular chaperones inside our cells are proteins that assist other cellular proteins in maintaining their structure-function relationship. These chaperones also feature in the FA assemblies, and emerging evidence is suggestive of their involvement in modulating focal adhesion dynamics as well. In addition to studies that have reported the role of chaperones in facilitating the folding and disaggregation of unfolded and aggregated proteins, results from Prof Shubhasis Haldar’s group at Ashoka University indicate molecular chaperones playing a mechanical role as well.
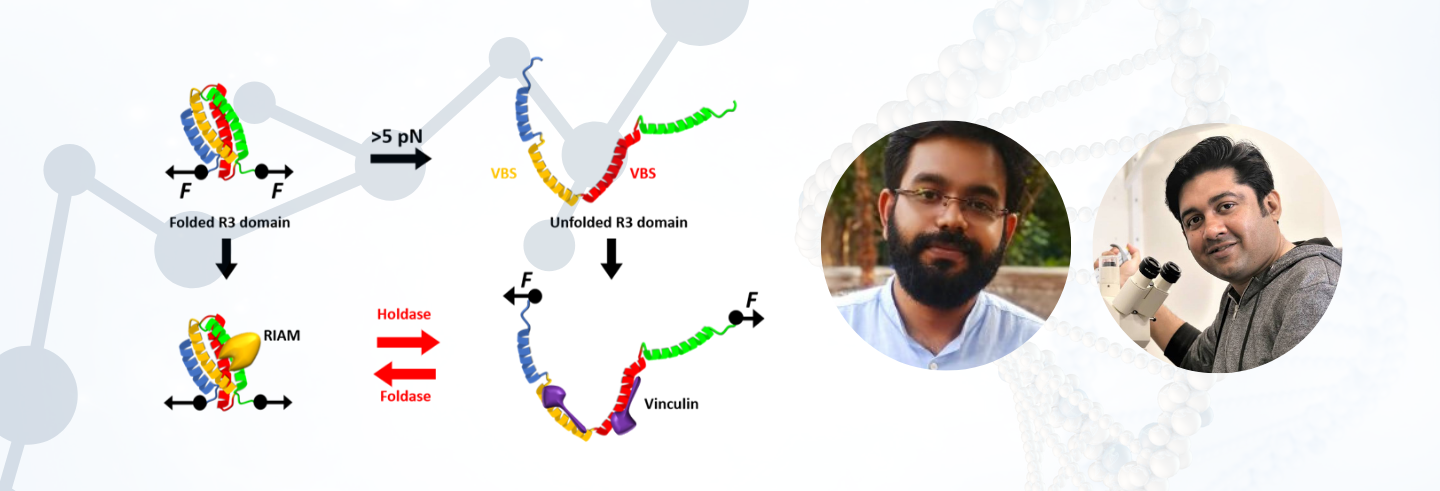
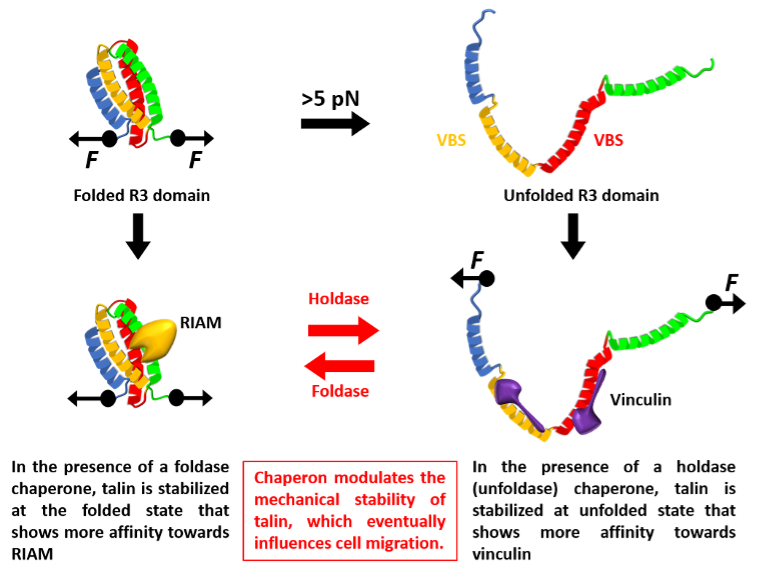
A single FA protein molecule tends to be force-sensitive within the piconewton (pN; 10-12 N) range, and molecular chaperones are able to modulate the force sensitivity of these FA proteins. Talin is a central FA protein that exhibits this force-dependent folding behaviour and consequent interactions. Within the 13 sub-domains (R1-R13) that make up its flexible rod domain, R3 is mechanically least stable, unfolding at a very low force of 5pN. The R13 domain, on the other hand, displays greater mechanical stability, unfolding at ~15 pN. Functionally, at forces <5pN, talin interacts with Rap1 Interacting Adaptor Molecule (RIAM), whereas at forces >5 pN, talin unfolds and interacts with vinculin. This mechanical switching is mirrored in cellular behaviour, accelerating cellular migration. Interestingly, Chakraborty, et al. have observed that molecular chaperones can modulate the mechanical stability of talin protein.
It was observed that holdase (unfoldase) chaperones – ones that help stabilize the protein in an unfolded state – facilitated unfolding of Talin at a lower force. In contrast, foldase chaperones increased the mechanical stability of talin, therefore unfolding at a higher force. Consequently, this chaperone-modulated mechanical stability can then influence FA complex dynamics and cellular behaviour.
In pathological conditions like metastatic cancer, where talin-centred FA dynamics play a central role, this force dependent interaction highlights a plausible mechanical effect of chaperones via triggering a domain-locked or domain-unlocked talin response in cell behaviour.
The Structural Mechanobiology Lab at Ashoka University has set up a key scientific instrument known as the Covalent Magnetic Tweezer (CMT) – a first of its kind in India – to understand this force-dependent interaction of talin with chaperones. This technique enables one to hold a single protein for extended durations of time. To put to scale, this technique operates on a single protein that is of the order of a billionth of the width of a single strand of hair.
Prepared by Soham Chakraborty, Ayush Mistry and Yukti Arora
Reference article: Direct observation of chaperone-modulated talin mechanics with single-molecule resolution, Communications Biology, Volume 5, Issue 1, April 4 2022
Authors of the research article:
Shubhasis Haldar* – Structural Mechanobiology Lab, Department of Biological Sciences, Ashoka University, Sonepat, Haryana, India
Soham Chakraborty – Department of Biological Sciences, Ashoka University, Sonipat, India
Deep Chaudhuri – Department of Biological Sciences, Ashoka University, Sonipat, India
Souradeep Banerjee – Department of Biological Sciences, Ashoka University, Sonipat, India
Madhu Bhatt – Department of Biological Sciences, Ashoka University, Sonipat, India
* Corresponding Author